To celebrate and promote World Cancer Day (February 4, 2022), CancerX hosted World Cancer Week 2022 from January 30 to February 5. This event involved 102 panelists, including experts and influencers from diver
Cancer Agenda
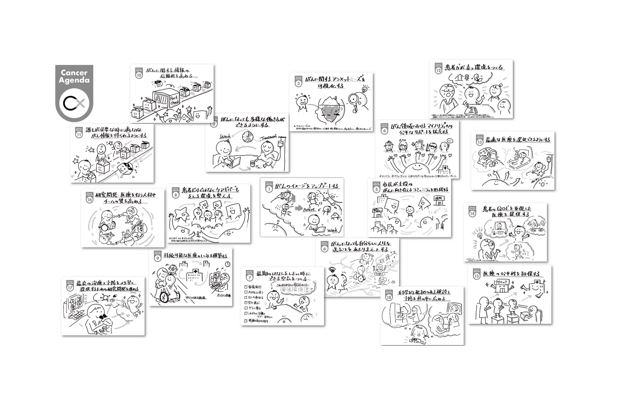
Cancer agenda visualizes the necessary and critical issues in order to move toward concrete solutions rather than a vague awareness of social problems. Eighteen items called the “Cancer Agenda” is an open resource that can be used by anyone.
- Improve the image of cancer.
- Enable people with cancer to work in diverse ways.
- Visualize unmet needs related to cancer.
- Provide equitable access to the members of minority groups with cancer-related issues.
- Create diverse communities within which laypeople with and without cancer play a leading role in supporting each other.
- Enable people to live their lives in their own way to the fullest extent possible despite a cancer diagnosis.
- Create a culture in which it is not taboo to talk about the final days.
- Create a culture supporting not only cancer patients but also caregivers.
- Build a sustainable healthcare system.
- Provide credible cancer information.
- Increase access to important cancer information and improve medical literacy.
- Create an environment that nurtures cancer patient empowerment.
- Enable cancer patients to choose the optimal medical treatment.
- Provide medical care that emphasizes the patient’s quality of life.
- Ensure the equity of cancer care.
- Invest in cancer healthcare providers and cancer researchers to improve the quality of team-based clinical care and research.
- Provide the best possible cancer treatment and prevention by promoting research and approval process.
- Promote the evidence-based cancer screening and prevention.
Two global sessions hosted by World Cancer Week 2022
CancerX Global
~How to close the care gap in developed countries~
Four panelists and a moderator who has extensive experience working internationally discussed ways to close the treatment gap in developed countries. Ms. Kuge’s wonderful facilitation led to interesting discussions on the issue of care gaps, such as differences in information and medical care received due to disparities in socioeconomic status and regional medical care infrastructure.
Dr. Ueno, a medical oncologist and a cancer survivor, explained the CancerX vision, working with UICC, and the importance of creating a comprehensive cancer treatment and prevention approach. Dr. Jean-Christophe Barland, working in the pharmaceutical industry, has worked in cross-functional collaboration to develop drugs for all cancer patients. Dr. Manago presented data from 1995 and 2018 to show that cancer mortality rates have decreased by 30% and 5-year survival rates have improved, signifying that cancer patients in Japan live longer. The Ministry of Health, Labour, and Welfare promotes cancer control measures.
The discussion focused on the importance of obtaining accurate information to close the care gap and, in the jumble of information on social networking sites, the importance of sharing best practices. Finally, Ms. Kuge summarized “the importance of creating a platform such as CancerX to promote exchanging ideas and sharing views related to care gaps from diverse people and social backgrounds. And the importance of mutual understanding of what gap issues are happening in different developed countries to close the care gap.
You can watch the archived session at the following link.
Archive link; https://link.cancerx.jp/wcw2022-023-global
CancerX R&D
~How we can accelerate the approval of cancer treatments~
The session featured three distinguished speakers who discussed the Pfizer and Moderna vaccine USA rapid approval process and how we can accelerate the approval process for cancer therapies. Under the Trump administration, Dr. Steve Hahn, who served as FDA Commissioner from 2019-2021, led Pfizer and Moderna’s expedited approval during the COVID-19 pandemic. He said, “The clinical trials and approval process that I experienced in the development of cancer drugs came into play in this expedited approval”.
The cooperation of the pharmaceutical companies is essential, and it is vital to ensure continuous communication and transparency of the approval process.” Dr. Yanagihara works as a review director reviewing oncology drugs at PMDA, the Japanese review organization. Dr. Yanagihara explained that Japan’s “Designation System for Pioneering Drugs” started in December 2019, which allowed the approval process in 6 months. Finally, Dr. Ohtsu, director of National Cancer Center Hospital East, reported on establishing a data platform for clinical trials involving hospitals and pharmaceutical companies across the country to create a cooperative system for the development of cancer drugs. After discussion, all panelists agree that there is a need for an expedited approval process for oncology similar to COVID-19 vaccine approval. Further, there is a need for establishing an international cooperative system with a transparent approval process. It is also vital to build an infrastructure that allows rapid participation in international joint clinical trials and sharing data.
You can watch the archived session at the following link.
Archive link; https://link.cancerx.jp/wcw2022-021-randd
About CancerX
CancerX is an organization that combines the power of people from diverse positions in industry, academia, government, and the private sector to work on solving social issues related to cancer. It was launched in 2018 and joined the UICC in 2020. As Japan’s largest platform for social issues related to cancer, it runs an annual conference and numerous subcommittees to address social agenda in conjunction with World Cancer Day.
– Co Representative Directors
Naoto T. Ueno (MD, PhD, FACP, The University of Texas MD Anderson Cancer Center, Professor of Medicine, Cancer survivor)
Elina Hanzawa (Producer, Dentsu Inc. / Editor-in-chief, cococolor)
– Directors
Rin Ogiya (MD, MPH, PhD, Government medical official, Ministry of Health, Labour and Welfare)
Akinori Kasuya (Sync Happiness Co., Ltd CEO Physical therapist Graduate School of Social Design Studies, Rikkyo University)
Yasutaka Kato (MD, PhD, Genomics Unit, Cancer Center, Keio University School of Medicine Assistant Professor)
Shuji Kitahara (Associate Professor Faculty of Advanced Techno-Surgery Institute of Advanced Biomedical Engineering and Science Tokyo Women’s Medical University / Executive Advisor to the City of Yokohama)
Misato Suzuki (St. Luke’s International Hospital, Medical Genetic Center, Certified Genetic Counselor)
Yuta Mishima (PhD, University of Tsukuba Faculty of Medicine, Trans-border Medical Research Center Department of Clinical Medicine Assistant Professor University of Tsukuba Hospital Office for the Promotion of Regenerative Medicine Deputy Director)
Noriko Moriuchi (DENTSU MEITETSU COMMUNICATIONS INC. Producer)
Mutsumi Yamagami (2020 J-TOP Workshop Vice-Chair / Certified Nurse Specialist in Cancer, The University of Tokyo Hospital)
*26 members including the above, and several volunteers.